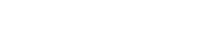Power from Water – How to produce oxygen and hydrogen on the Moon
In this set of three activities, students will learn about electrochemistry.
In the first activity, they will build a voltaic pile – a simple battery. This invention marked the beginning of electrochemistry.
Students will then study electrolysis. Electrolysis uses electric current to split water into its components: hydrogen and oxygen. These products can be used as propellants for spacecraft and/or to provide oxygen to support a crew.
In the last activity, students examine and use a fuel cell.
Learning Objectives
Age range:
14 – 16 years old
Time
Preparation: 1 hour
Lesson: 2 hours
Lesson: 2 hours
Resource available in:
Activity 1: Build your own battery
In this activity, students will construct a voltaic pile – a simple battery – from metal plates, dishcloth and vinegar. A voltaic pile uses a spontaneous chemical reaction to create electricity.
Equipment
Activity 2: Electrolysis
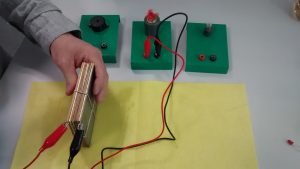
In this activity, students will build an electrolyser: a device that introduces an electric current into a liquid using two electrodes. They will use the device for water electrolysis and discover that it is possible to split water into its components: oxygen and hydrogen.
Equipment
Activity 3: Fuel cell
In this activity, students will use the products of water electrolysis (H2 and O2) in a fuel cell. They will investigate how fuel cells produce electricity and heat from a chemical reaction. Students will consider the possibilities and limitations of fuel cells for Moon exploration.
Equipment
Did you know?
Electrolysis of water is the main method of generating oxygen on board the International Space Station (ISS). Water is collected from urine, wastewater, and condensation and split into oxygen and hydrogen in the Oxygen Generation System (OGS).
The station’s football-field-sized solar arrays are the power source. A similar system could be used on the Moon.
The station’s football-field-sized solar arrays are the power source. A similar system could be used on the Moon.

The ISS orbiting Earth
Landing on the moon – Planning and designing a lunar lander
Brief description: In this set of activities, students will plan, design and build a landing module to secure the survival of the crew (in the

Mission on the Moon – Program a classmate to complete a mission on the Moon
Brief description: This activity will introduce students to logical thinking by planning, testing and executing a simple mission on the Moon. Students will work in

Moon Constitution – How would a future lunar community be organised?
Brief description: In this resource, pupils will debate some organisational and social characteristics of a future settlement on the Moon and relate it to their